
AC T16715
XX
ID T16715
XX
DT 25.09.2009 (created); smt.
DT 11.12.2015 (updated); sup.
CO Copyright (C), QIAGEN.
XX
FA RXR-gamma
XX
SY NR2B3; retinoid X receptor gamma; RXR-gamma; RXRG.
XX
OS mouse, Mus musculus
OC eukaryota; animalia; metazoa; chordata; vertebrata; tetrapoda; mammalia; eutheria; rodentia; myomorpha; muridae; murinae
XX
GE G006696 Rxrg.
XX
CL C0002; CC (rec).
XX
SZ 463 AA; 50.9 kDa (cDNA) (calc.).
XX
SQ MYGNYSHFMKFPTGFGGSPGHTGSTSMSPSVALPTGKPMDSHPSYTDTPVSAPRTLSAVG
SQ TPLNALGSPYRVITSAMGPPSGALAAPPGINLVAPPSSQLNVVNSVSSSEDIKPLPGLPG
SQ IGNMNYPSTSPGSLVKHICAICGDRSSGKHYGVYSCEGCKGFFKRTIRKDLIYTCRDNKD
SQ CLIDKRQRNRCQYCRYQKCLVMGMKREAVQEERQRSRERAESEAECASSSHEDMPVERIL
SQ EAELAVEPKTESYGDMNVENSTNDPVTNICHAADKQLFTLVEWAKRIPHFSDLTLEDQVI
SQ LLRAGWNELLIASFSHRSVSVQDGILLATGLHVHRSSAHSAGVGSIFDRVLTELVSKMKD
SQ MQMDKSELGCLRAIVLFNPDAKGLSNPSEVETLREKVYATLEAYTKQKYPEQPGRFAKLL
SQ LRLPALRSIGLKCLEHLFFFKLIGDTPIDSFLMEMLETPLQIT
XX
SC Swiss-Prot#P28705
XX
FT 1 138  region A/B (modulating transactivation functions) [3].
FT 37 411
region A/B (modulating transactivation functions) [3].
FT 37 411  PF00478; IMP dehydrogenase / GMP reductase domai.
FT 136 207
PF00478; IMP dehydrogenase / GMP reductase domai.
FT 136 207  SM00399; c4gold.
FT 136 211
SM00399; c4gold.
FT 136 211  PS51030; NUCLEAR_REC_DBD_2.
FT 137 212
PS51030; NUCLEAR_REC_DBD_2.
FT 137 212  PF00105; Zinc finger, C4 type (two domains).
FT 139 204
PF00105; Zinc finger, C4 type (two domains).
FT 139 204  region C (DNA-binding, dimerization) [3].
FT 205 213
region C (DNA-binding, dimerization) [3].
FT 205 213  T-box, functional: important for dimerization with T01354 [7].
FT 205 228
T-box, functional: important for dimerization with T01354 [7].
FT 205 228  region D (hinge region) [3].
FT 229 463
region D (hinge region) [3].
FT 229 463  region E/F (ligand-binding, dimerization, activating function 2) [3].
FT 271 430
region E/F (ligand-binding, dimerization, activating function 2) [3].
FT 271 430  SM00430; holi.
FT 274 455
SM00430; holi.
FT 274 455  PF00104; Ligand-binding domain of nuclear hormon.
PF00104; Ligand-binding domain of nuclear hormon.
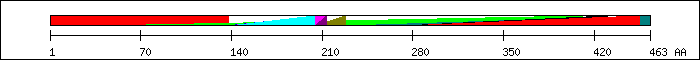 XX
IN T08888 LXRalpha-isoform1; human, Homo sapiens.
XX
MX M00963 V$T3R_Q6.
XX
BS R03935.
BS R03936.
BS R08504.
BS R11727.
XX
DR TRANSPATH: MO000161932.
DR EMBL: M84819;
DR EMBL: X66225;
DR UniProtKB: P28705;
XX
RN [1]; RE0000265.
RX PUBMED: 1310259.
RA Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J.-Y., Staub A., Garnier J.-M., Mader S., Chambon P.
RT Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently
RL Cell 68:377-395 (1992).
RN [2]; RE0000270.
RX PUBMED: 1326406.
RA Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P.
RT Promoter context-and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors
RL Cell 70:1007-1019 (1992).
RN [3]; RE0000789.
RX PUBMED: 1312497.
RA Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M.
RT Characterization of three RXR genes that mediate the action of 9-cis retinoic acid
RL Genes Dev. 6:329-344 (1992).
RN [4]; RE0002413.
RX PUBMED: 2554307.
RA Hamada K., Gleason S., Levi B.-Z., Hirschfeld S., Apella E., Ozato K.
RT H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element
RL Proc. Natl. Acad. Sci. USA 86:8289-8293 (1989).
RN [5]; RE0003240.
RX PUBMED: 8127707.
RA Muscat G. E. O., Mynett-Johnson L., Dowhan D., Downes M., Griggs R.
RT Activation of MyoD gene transcription by 3, 4, 3'-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors
RL Nucleic Acids Res. 22:583-591 (1994).
RN [6]; RE0013625.
RX PUBMED: 10219237.
RA Nuclear Receptors Nomenclature Committee.
RT A unified nomenclature system for the nuclear receptor superfamily
RL Cell 97:161-163 (1999).
RN [7]; RE0017065.
RX PUBMED: 9242684.
RA IJpenberg A., Jeannin E., Wahli W., Desvergne B.
RT Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element
RL J. Biol. Chem. 272:20108-20117 (1997).
XX
//
XX
IN T08888 LXRalpha-isoform1; human, Homo sapiens.
XX
MX M00963 V$T3R_Q6.
XX
BS R03935.
BS R03936.
BS R08504.
BS R11727.
XX
DR TRANSPATH: MO000161932.
DR EMBL: M84819;
DR EMBL: X66225;
DR UniProtKB: P28705;
XX
RN [1]; RE0000265.
RX PUBMED: 1310259.
RA Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J.-Y., Staub A., Garnier J.-M., Mader S., Chambon P.
RT Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently
RL Cell 68:377-395 (1992).
RN [2]; RE0000270.
RX PUBMED: 1326406.
RA Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P.
RT Promoter context-and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors
RL Cell 70:1007-1019 (1992).
RN [3]; RE0000789.
RX PUBMED: 1312497.
RA Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M.
RT Characterization of three RXR genes that mediate the action of 9-cis retinoic acid
RL Genes Dev. 6:329-344 (1992).
RN [4]; RE0002413.
RX PUBMED: 2554307.
RA Hamada K., Gleason S., Levi B.-Z., Hirschfeld S., Apella E., Ozato K.
RT H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element
RL Proc. Natl. Acad. Sci. USA 86:8289-8293 (1989).
RN [5]; RE0003240.
RX PUBMED: 8127707.
RA Muscat G. E. O., Mynett-Johnson L., Dowhan D., Downes M., Griggs R.
RT Activation of MyoD gene transcription by 3, 4, 3'-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors
RL Nucleic Acids Res. 22:583-591 (1994).
RN [6]; RE0013625.
RX PUBMED: 10219237.
RA Nuclear Receptors Nomenclature Committee.
RT A unified nomenclature system for the nuclear receptor superfamily
RL Cell 97:161-163 (1999).
RN [7]; RE0017065.
RX PUBMED: 9242684.
RA IJpenberg A., Jeannin E., Wahli W., Desvergne B.
RT Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element
RL J. Biol. Chem. 272:20108-20117 (1997).
XX
//


region A/B (modulating transactivation functions) [3]. FT 37 411
PF00478; IMP dehydrogenase / GMP reductase domai. FT 136 207
SM00399; c4gold. FT 136 211
PS51030; NUCLEAR_REC_DBD_2. FT 137 212
PF00105; Zinc finger, C4 type (two domains). FT 139 204
region C (DNA-binding, dimerization) [3]. FT 205 213
T-box, functional: important for dimerization with T01354 [7]. FT 205 228
region D (hinge region) [3]. FT 229 463
region E/F (ligand-binding, dimerization, activating function 2) [3]. FT 271 430
SM00430; holi. FT 274 455
PF00104; Ligand-binding domain of nuclear hormon.
XX IN T08888 LXRalpha-isoform1; human, Homo sapiens. XX MX M00963 V$T3R_Q6. XX BS R03935. BS R03936. BS R08504. BS R11727. XX DR TRANSPATH: MO000161932. DR EMBL: M84819; DR EMBL: X66225; DR UniProtKB: P28705; XX RN [1]; RE0000265. RX PUBMED: 1310259. RA Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J.-Y., Staub A., Garnier J.-M., Mader S., Chambon P. RT Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently RL Cell 68:377-395 (1992). RN [2]; RE0000270. RX PUBMED: 1326406. RA Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. RT Promoter context-and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors RL Cell 70:1007-1019 (1992). RN [3]; RE0000789. RX PUBMED: 1312497. RA Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. RT Characterization of three RXR genes that mediate the action of 9-cis retinoic acid RL Genes Dev. 6:329-344 (1992). RN [4]; RE0002413. RX PUBMED: 2554307. RA Hamada K., Gleason S., Levi B.-Z., Hirschfeld S., Apella E., Ozato K. RT H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element RL Proc. Natl. Acad. Sci. USA 86:8289-8293 (1989). RN [5]; RE0003240. RX PUBMED: 8127707. RA Muscat G. E. O., Mynett-Johnson L., Dowhan D., Downes M., Griggs R. RT Activation of MyoD gene transcription by 3, 4, 3'-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors RL Nucleic Acids Res. 22:583-591 (1994). RN [6]; RE0013625. RX PUBMED: 10219237. RA Nuclear Receptors Nomenclature Committee. RT A unified nomenclature system for the nuclear receptor superfamily RL Cell 97:161-163 (1999). RN [7]; RE0017065. RX PUBMED: 9242684. RA IJpenberg A., Jeannin E., Wahli W., Desvergne B. RT Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element RL J. Biol. Chem. 272:20108-20117 (1997). XX //