
AC T04378
XX
ID T04378
XX
DT 13.03.2001 (created); dkl.
DT 27.03.2014 (updated); pro.
CO Copyright (C), QIAGEN.
XX
FA Mad
XX
SY CG12399; mothers against decapentaplegic.
XX
OS fruit fly, Drosophila melanogaster
OC eukaryota; animalia; metazoa; arthropoda; insecta; diptera; drosophiloidea; drosophilidae
XX
GE G002362 Mad.
XX
CL C0041; SMAD.
XX
SZ 455 AA; 50.5 kDa (cDNA) (calc.).
XX
SQ MDTDDVESNTSSAMSTLGSLFSFTSPAVKKLLGWKQGDEEEKWAEKAVDSLVKKLKKRKG
SQ AIEELERALSCPGQPSKCVTIPRSLDGRLQVSHRKGLPHVIYCRVWRWPDLQSHHELKPL
SQ ELCQYPFSAKQKEVCINPYHYKRVESPVLPPVLVPRHSEFAPGHSMLQFNHVAEPSMPHN
SQ VSYSNSGFNSHSLSTSNTSVGSPSSVNSNPNSPYDSLAGTPPPAYSPSEDGNSNNPNDGG
SQ QLLDAQMGDVAQVSYSEPAFWASIAYYELNCRVGEVFHCNNNSVIVDGFTNPSNNSDRCC
SQ LGQLSNVNRNSTIENTRRHIGKGVHLYYVTGEVYAECLSDSAIFVQSRNCNYHHGFHPST
SQ VCKIPPGCSLKIFNNQEFAQLLSQSVNNGFEAVYELTKMCTIRMSFVKGWGAEYHRQDVT
SQ STPCWIEIHLHGPLQWLDKVLTQMGSPHNAISSVS
XX
SC translated from EMBL #U10328
XX
FT 20 159  MH1 domain [2].
FT 26 150
MH1 domain [2].
FT 26 150  PS51075; MH1.
FT 39 148
PS51075; MH1.
FT 39 148  SM00523; dwAneu5.
FT 41 145
SM00523; dwAneu5.
FT 41 145  PF03165; MH1 domain.
FT 53 57
PF03165; MH1 domain.
FT 53 57  Nuclear Localization Signal (NLS) [6].
FT 220 225
Nuclear Localization Signal (NLS) [6].
FT 220 225  PY motif [2].
FT 255 433
PY motif [2].
FT 255 433  PF03166; MH2 domain.
FT 259 431
PF03166; MH2 domain.
FT 259 431  SM00524; DWB.
FT 261 451
SM00524; DWB.
FT 261 451  MH2 domain [2].
FT 261 455
MH2 domain [2].
FT 261 455  PS51076; MH2.
FT 452 455
PS51076; MH2.
FT 452 455  SSXS motif [2].
SSXS motif [2].
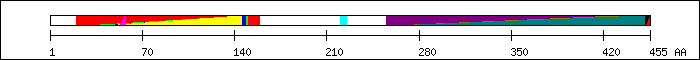 XX
SF NLS (Nuclear localization Signal) determines the ligand-induced nuclear translocation [6];
XX
FF interacts with Shn (Schnurri) T02332 in the presence of TkvA (Dpp Type-1 receptor) [7];
FF MH2 domain + Linker region is sufficient for interaction with Shn T02332 [7];
FF C-terminal domain of CBP (AA2240-AA2608, CREB Binding Protein) T03236 interacts with MH2 domain [8];
FF in the presence of PUNT (receptor-type II) and TKV (Thickvein, receptor-type I) phosphorylation is induced [5] [9];
FF addition of BMP-2 enhanced phosphorylation [5];
FF Mad protein was detected throughout the cell in the absence of signal, coexpression with activated TKV receptor resulted in accumulation of Mad in the nucleus [4];
FF phosphorylation is essential for accumulation in the nucleus [4];
FF DAD inhibits phosphorylation of Mad by TKV by competing with Mad in association with the receptor [5];
XX
MX M07148 I$MAD_01.
MX M01090 I$MAD_Q6.
XX
BS R10037.
BS R36651.
BS R36656.
BS R36657.
BS R60983.
BS R10014.
BS R10010.
BS R10100.
BS R10008.
BS R17894.
BS R17895.
BS R17896.
BS R17899.
BS R17901.
BS R17903.
BS R17904.
BS R17905.
BS R17906.
BS R17912.
XX
DR TRANSPATH: MO000019014.
DR EMBL: U10328;
DR UniProtKB: P42003;
DR FLYBASE: FBgn0011648.
XX
RN [1]; RE0014837.
RX PUBMED: 10731132.
RA Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F., George R. A., Lewis S. E., Richards S., Ashburner M., Henderson S. N., Sutton G. G., Wortman J. R., Yandell M. D., Zhang Q., Chen L. X., Brandon R. C., Rogers Y. H., Blazej R. G., Champe M., Pfeiffer B. D., Wan K. H., Doyle C., Baxter E. G., Helt G., Nelson C. R., Gabor Miklos G. L., Abril J. F., Agbayani A., An H. J., Andrews-Pfannkoch C., Baldwin D., Ballew R. M., Basu A., Baxendale J., Bayraktaroglu L., Beasley E. M., Beeson K. Y., Benos P. V., Berman B. P., Bhandari D., Bolshakov S., Borkova D., Botchan M. R., Bouck J., Brokstein P., Brottier P., Burtis K. C., Busam D. A., Butler H., Cadieu E., Center A., Chandra I., Cherry J. M., Cawley S., Dahlke C., Davenport L. B., Davies P., de Pablos B., Delcher A., Deng Z., Mays A. D., Dew I., Dietz S. M., Dodson K., Doup L. E., Downes M., Dugan-Rocha S., Dunkov B. C., Dunn P., Durbin K. J., Evangelista C. C., Ferraz C., Ferriera S., Fleischmann W., Fosler C., Gabrielian A. E., Garg N. S., Gelbart W. M., Glasser K., Glodek A., Gong F., Gorrell J. H., Gu Z., Guan P., Harris M., Harris N. L., Harvey D., Heiman T. J., Hernandez J. R., Houck J., Hostin D., Houston K. A., Howland T. J., Wei M. H., Ibegwam C., Jalali M., Kalush F., Karpen G. H., Ke Z., Kennison J. A., Ketchum K. A., Kimmel B. E., Kodira C. D., Kraft C., Kravitz S., Kulp D., Lai Z., Lasko P., Lei Y., Levitsky A. A., Li J., Li Z., Liang Y., Lin X., Liu X., Mattei B., McIntosh T. C., McLeod M. P., McPherson D., Merkulov G., Milshina N. V., Mobarry C., Morris J., Moshrefi A., Mount S. M., Moy M., Murphy B., Murphy L., Muzny D. M., Nelson D. L., Nelson D. R., Nelson K. A., Nixon K., Nusskern D. R., Pacleb J. M., Palazzolo M., Pittman G. S., Pan S., Pollard J., Puri V., Reese M. G., Reinert K., Remington K., Saunders R. D., Scheeler F., Shen H., Shue B. C., Siden-Kiamos I., Simpson M., Skupski M. P., Smith T., Spier E., Spradling A. C., Stapleton M., Strong R., Sun E., Svirskas R., Tector C., Turner R., Venter E., Wang A. H., Wang X., Wang Z. Y., Wassarman D. A., Weinstock G. M., Weissenbach J., Williams S. M., Woodage T., Worley K. C., Wu D., Yang S., Yao Q. A., Ye J., Yeh R. F., Zaveri J. S., Zhan M., Zhang G., Zhao Q., Zheng L., Zheng X. H., Zhong F. N., Zhong W., Zhou X., Zhu S., Zhu X., Smith H. O., Gibbs R. A., Myers E. W., Rubin G. M., Venter J. C.
RT The genome sequence of Drosophila melanogaster
RL Science 287:2185-2195 (2000).
RN [2]; RE0015616.
RX PUBMED: 9887103.
RA Brummel T., Abdollah S., Haerry T. E., Shimell M. J., Merriam J., Raftery L., Wrana J. L., O'Connor M. B.
RT The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development
RL Genes Dev. 13:98-111 (1999).
RN [3]; RE0015898.
RX PUBMED: 7768443.
RA Sekelsky J. J., Newfeld S. J., Raftery L. A., Chartoff E. H., Gelbart W. M.
RT Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster.
RL Genetics 139:1347-1358 (1995).
RN [4]; RE0015907.
RX PUBMED: 9502724.
RA Wisotzkey R. G., Mehra A., Sutherland D. J., Dobens L. L., Liu X., Dohrmann C., Attisano L., Raftery L. A.
RT Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses.
RL Development 125:1433-1445 (1998).
RN [5]; RE0015915.
RX PUBMED: 9693372.
RA Inoue H., Imamura T., Ishidou Y., Takase M., Udagawa Y., Oka Y., Tsuneizumi K., Tabata T., Miyazono K., Kawabata M.
RT Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp.
RL Mol. Biol. Cell 9:2145-2156 (1998).
RN [6]; RE0016203.
RX PUBMED: 10884415.
RA Xiao Z., Liu X., Henis Y. I., Lodish H. F.
RT A distinct nuclear localization signal in the N terminus of Smad 3 determines its ligand-induced nuclear translocation.
RL Proc. Natl. Acad. Sci. USA 97:7853-7858 (2000).
RN [7]; RE0016241.
RX PUBMED: 11071761.
RA Dai H., Hogan C., Gopalakrishnan B., Torres-Vazquez J., Nguyen M., Park S., Raftery L. A., Warrior R., Arora K.
RT The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic.
RL Dev. Biol. 227:373-387 (2000).
RN [8]; RE0016258.
RX PUBMED: 10075933.
RA Waltzer L., Bienz M.
RT A function of CBP as a transcriptional co-activator during Dpp signalling.
RL EMBO J. 18:1630-1641 (1999).
RN [9]; RE0016261.
RX PUBMED: 10320478.
RA Das P., Inoue H., Baker J. C., Beppu H., Kawabata M., Harland R. M., Miyazono K., Padgett R. W.
RT Drosophila dSmad2 and Atr-I transmit activin/TGFbeta signals.
RL Genes Cells 4:123-134 (1999).
RN [10]; RE0022036.
RX PUBMED: 10370243.
RA Zhang Y., Derynck R.
RT Regulation of Smad signalling by protein associations and signalling crosstalk
RL Trends Cell Biol. 9:274-279 (1999).
RN [11]; RE0022065.
RX PUBMED: 10712925.
RA Attisano L., Wrana J. L.
RT Smads as transcriptional co-modulators
RL Curr. Opin. Cell Biol. 12:235-243 (2000).
RN [12]; RE0022070.
RX PUBMED: 9529613.
RA Kretzschmar M., Massague J.
RT SMADs: mediators and regulators of TGF-beta signaling
RL Curr. Opin. Gen. Dev. 8:103-111 (1998).
RN [13]; RE0022072.
RX PUBMED: 10831835.
RA Zimmerman C. M., Padgett R. W.
RT Transforming growth factor beta signaling mediators and modulators
RL Gene 249:17-30 (2000).
XX
//
XX
SF NLS (Nuclear localization Signal) determines the ligand-induced nuclear translocation [6];
XX
FF interacts with Shn (Schnurri) T02332 in the presence of TkvA (Dpp Type-1 receptor) [7];
FF MH2 domain + Linker region is sufficient for interaction with Shn T02332 [7];
FF C-terminal domain of CBP (AA2240-AA2608, CREB Binding Protein) T03236 interacts with MH2 domain [8];
FF in the presence of PUNT (receptor-type II) and TKV (Thickvein, receptor-type I) phosphorylation is induced [5] [9];
FF addition of BMP-2 enhanced phosphorylation [5];
FF Mad protein was detected throughout the cell in the absence of signal, coexpression with activated TKV receptor resulted in accumulation of Mad in the nucleus [4];
FF phosphorylation is essential for accumulation in the nucleus [4];
FF DAD inhibits phosphorylation of Mad by TKV by competing with Mad in association with the receptor [5];
XX
MX M07148 I$MAD_01.
MX M01090 I$MAD_Q6.
XX
BS R10037.
BS R36651.
BS R36656.
BS R36657.
BS R60983.
BS R10014.
BS R10010.
BS R10100.
BS R10008.
BS R17894.
BS R17895.
BS R17896.
BS R17899.
BS R17901.
BS R17903.
BS R17904.
BS R17905.
BS R17906.
BS R17912.
XX
DR TRANSPATH: MO000019014.
DR EMBL: U10328;
DR UniProtKB: P42003;
DR FLYBASE: FBgn0011648.
XX
RN [1]; RE0014837.
RX PUBMED: 10731132.
RA Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F., George R. A., Lewis S. E., Richards S., Ashburner M., Henderson S. N., Sutton G. G., Wortman J. R., Yandell M. D., Zhang Q., Chen L. X., Brandon R. C., Rogers Y. H., Blazej R. G., Champe M., Pfeiffer B. D., Wan K. H., Doyle C., Baxter E. G., Helt G., Nelson C. R., Gabor Miklos G. L., Abril J. F., Agbayani A., An H. J., Andrews-Pfannkoch C., Baldwin D., Ballew R. M., Basu A., Baxendale J., Bayraktaroglu L., Beasley E. M., Beeson K. Y., Benos P. V., Berman B. P., Bhandari D., Bolshakov S., Borkova D., Botchan M. R., Bouck J., Brokstein P., Brottier P., Burtis K. C., Busam D. A., Butler H., Cadieu E., Center A., Chandra I., Cherry J. M., Cawley S., Dahlke C., Davenport L. B., Davies P., de Pablos B., Delcher A., Deng Z., Mays A. D., Dew I., Dietz S. M., Dodson K., Doup L. E., Downes M., Dugan-Rocha S., Dunkov B. C., Dunn P., Durbin K. J., Evangelista C. C., Ferraz C., Ferriera S., Fleischmann W., Fosler C., Gabrielian A. E., Garg N. S., Gelbart W. M., Glasser K., Glodek A., Gong F., Gorrell J. H., Gu Z., Guan P., Harris M., Harris N. L., Harvey D., Heiman T. J., Hernandez J. R., Houck J., Hostin D., Houston K. A., Howland T. J., Wei M. H., Ibegwam C., Jalali M., Kalush F., Karpen G. H., Ke Z., Kennison J. A., Ketchum K. A., Kimmel B. E., Kodira C. D., Kraft C., Kravitz S., Kulp D., Lai Z., Lasko P., Lei Y., Levitsky A. A., Li J., Li Z., Liang Y., Lin X., Liu X., Mattei B., McIntosh T. C., McLeod M. P., McPherson D., Merkulov G., Milshina N. V., Mobarry C., Morris J., Moshrefi A., Mount S. M., Moy M., Murphy B., Murphy L., Muzny D. M., Nelson D. L., Nelson D. R., Nelson K. A., Nixon K., Nusskern D. R., Pacleb J. M., Palazzolo M., Pittman G. S., Pan S., Pollard J., Puri V., Reese M. G., Reinert K., Remington K., Saunders R. D., Scheeler F., Shen H., Shue B. C., Siden-Kiamos I., Simpson M., Skupski M. P., Smith T., Spier E., Spradling A. C., Stapleton M., Strong R., Sun E., Svirskas R., Tector C., Turner R., Venter E., Wang A. H., Wang X., Wang Z. Y., Wassarman D. A., Weinstock G. M., Weissenbach J., Williams S. M., Woodage T., Worley K. C., Wu D., Yang S., Yao Q. A., Ye J., Yeh R. F., Zaveri J. S., Zhan M., Zhang G., Zhao Q., Zheng L., Zheng X. H., Zhong F. N., Zhong W., Zhou X., Zhu S., Zhu X., Smith H. O., Gibbs R. A., Myers E. W., Rubin G. M., Venter J. C.
RT The genome sequence of Drosophila melanogaster
RL Science 287:2185-2195 (2000).
RN [2]; RE0015616.
RX PUBMED: 9887103.
RA Brummel T., Abdollah S., Haerry T. E., Shimell M. J., Merriam J., Raftery L., Wrana J. L., O'Connor M. B.
RT The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development
RL Genes Dev. 13:98-111 (1999).
RN [3]; RE0015898.
RX PUBMED: 7768443.
RA Sekelsky J. J., Newfeld S. J., Raftery L. A., Chartoff E. H., Gelbart W. M.
RT Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster.
RL Genetics 139:1347-1358 (1995).
RN [4]; RE0015907.
RX PUBMED: 9502724.
RA Wisotzkey R. G., Mehra A., Sutherland D. J., Dobens L. L., Liu X., Dohrmann C., Attisano L., Raftery L. A.
RT Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses.
RL Development 125:1433-1445 (1998).
RN [5]; RE0015915.
RX PUBMED: 9693372.
RA Inoue H., Imamura T., Ishidou Y., Takase M., Udagawa Y., Oka Y., Tsuneizumi K., Tabata T., Miyazono K., Kawabata M.
RT Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp.
RL Mol. Biol. Cell 9:2145-2156 (1998).
RN [6]; RE0016203.
RX PUBMED: 10884415.
RA Xiao Z., Liu X., Henis Y. I., Lodish H. F.
RT A distinct nuclear localization signal in the N terminus of Smad 3 determines its ligand-induced nuclear translocation.
RL Proc. Natl. Acad. Sci. USA 97:7853-7858 (2000).
RN [7]; RE0016241.
RX PUBMED: 11071761.
RA Dai H., Hogan C., Gopalakrishnan B., Torres-Vazquez J., Nguyen M., Park S., Raftery L. A., Warrior R., Arora K.
RT The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic.
RL Dev. Biol. 227:373-387 (2000).
RN [8]; RE0016258.
RX PUBMED: 10075933.
RA Waltzer L., Bienz M.
RT A function of CBP as a transcriptional co-activator during Dpp signalling.
RL EMBO J. 18:1630-1641 (1999).
RN [9]; RE0016261.
RX PUBMED: 10320478.
RA Das P., Inoue H., Baker J. C., Beppu H., Kawabata M., Harland R. M., Miyazono K., Padgett R. W.
RT Drosophila dSmad2 and Atr-I transmit activin/TGFbeta signals.
RL Genes Cells 4:123-134 (1999).
RN [10]; RE0022036.
RX PUBMED: 10370243.
RA Zhang Y., Derynck R.
RT Regulation of Smad signalling by protein associations and signalling crosstalk
RL Trends Cell Biol. 9:274-279 (1999).
RN [11]; RE0022065.
RX PUBMED: 10712925.
RA Attisano L., Wrana J. L.
RT Smads as transcriptional co-modulators
RL Curr. Opin. Cell Biol. 12:235-243 (2000).
RN [12]; RE0022070.
RX PUBMED: 9529613.
RA Kretzschmar M., Massague J.
RT SMADs: mediators and regulators of TGF-beta signaling
RL Curr. Opin. Gen. Dev. 8:103-111 (1998).
RN [13]; RE0022072.
RX PUBMED: 10831835.
RA Zimmerman C. M., Padgett R. W.
RT Transforming growth factor beta signaling mediators and modulators
RL Gene 249:17-30 (2000).
XX
//


MH1 domain [2]. FT 26 150
PS51075; MH1. FT 39 148
SM00523; dwAneu5. FT 41 145
PF03165; MH1 domain. FT 53 57
Nuclear Localization Signal (NLS) [6]. FT 220 225
PY motif [2]. FT 255 433
PF03166; MH2 domain. FT 259 431
SM00524; DWB. FT 261 451
MH2 domain [2]. FT 261 455
PS51076; MH2. FT 452 455
SSXS motif [2].
XX SF NLS (Nuclear localization Signal) determines the ligand-induced nuclear translocation [6]; XX FF interacts with Shn (Schnurri) T02332 in the presence of TkvA (Dpp Type-1 receptor) [7]; FF MH2 domain + Linker region is sufficient for interaction with Shn T02332 [7]; FF C-terminal domain of CBP (AA2240-AA2608, CREB Binding Protein) T03236 interacts with MH2 domain [8]; FF in the presence of PUNT (receptor-type II) and TKV (Thickvein, receptor-type I) phosphorylation is induced [5] [9]; FF addition of BMP-2 enhanced phosphorylation [5]; FF Mad protein was detected throughout the cell in the absence of signal, coexpression with activated TKV receptor resulted in accumulation of Mad in the nucleus [4]; FF phosphorylation is essential for accumulation in the nucleus [4]; FF DAD inhibits phosphorylation of Mad by TKV by competing with Mad in association with the receptor [5]; XX MX M07148 I$MAD_01. MX M01090 I$MAD_Q6. XX BS R10037. BS R36651. BS R36656. BS R36657. BS R60983. BS R10014. BS R10010. BS R10100. BS R10008. BS R17894. BS R17895. BS R17896. BS R17899. BS R17901. BS R17903. BS R17904. BS R17905. BS R17906. BS R17912. XX DR TRANSPATH: MO000019014. DR EMBL: U10328; DR UniProtKB: P42003; DR FLYBASE: FBgn0011648. XX RN [1]; RE0014837. RX PUBMED: 10731132. RA Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F., George R. A., Lewis S. E., Richards S., Ashburner M., Henderson S. N., Sutton G. G., Wortman J. R., Yandell M. D., Zhang Q., Chen L. X., Brandon R. C., Rogers Y. H., Blazej R. G., Champe M., Pfeiffer B. D., Wan K. H., Doyle C., Baxter E. G., Helt G., Nelson C. R., Gabor Miklos G. L., Abril J. F., Agbayani A., An H. J., Andrews-Pfannkoch C., Baldwin D., Ballew R. M., Basu A., Baxendale J., Bayraktaroglu L., Beasley E. M., Beeson K. Y., Benos P. V., Berman B. P., Bhandari D., Bolshakov S., Borkova D., Botchan M. R., Bouck J., Brokstein P., Brottier P., Burtis K. C., Busam D. A., Butler H., Cadieu E., Center A., Chandra I., Cherry J. M., Cawley S., Dahlke C., Davenport L. B., Davies P., de Pablos B., Delcher A., Deng Z., Mays A. D., Dew I., Dietz S. M., Dodson K., Doup L. E., Downes M., Dugan-Rocha S., Dunkov B. C., Dunn P., Durbin K. J., Evangelista C. C., Ferraz C., Ferriera S., Fleischmann W., Fosler C., Gabrielian A. E., Garg N. S., Gelbart W. M., Glasser K., Glodek A., Gong F., Gorrell J. H., Gu Z., Guan P., Harris M., Harris N. L., Harvey D., Heiman T. J., Hernandez J. R., Houck J., Hostin D., Houston K. A., Howland T. J., Wei M. H., Ibegwam C., Jalali M., Kalush F., Karpen G. H., Ke Z., Kennison J. A., Ketchum K. A., Kimmel B. E., Kodira C. D., Kraft C., Kravitz S., Kulp D., Lai Z., Lasko P., Lei Y., Levitsky A. A., Li J., Li Z., Liang Y., Lin X., Liu X., Mattei B., McIntosh T. C., McLeod M. P., McPherson D., Merkulov G., Milshina N. V., Mobarry C., Morris J., Moshrefi A., Mount S. M., Moy M., Murphy B., Murphy L., Muzny D. M., Nelson D. L., Nelson D. R., Nelson K. A., Nixon K., Nusskern D. R., Pacleb J. M., Palazzolo M., Pittman G. S., Pan S., Pollard J., Puri V., Reese M. G., Reinert K., Remington K., Saunders R. D., Scheeler F., Shen H., Shue B. C., Siden-Kiamos I., Simpson M., Skupski M. P., Smith T., Spier E., Spradling A. C., Stapleton M., Strong R., Sun E., Svirskas R., Tector C., Turner R., Venter E., Wang A. H., Wang X., Wang Z. Y., Wassarman D. A., Weinstock G. M., Weissenbach J., Williams S. M., Woodage T., Worley K. C., Wu D., Yang S., Yao Q. A., Ye J., Yeh R. F., Zaveri J. S., Zhan M., Zhang G., Zhao Q., Zheng L., Zheng X. H., Zhong F. N., Zhong W., Zhou X., Zhu S., Zhu X., Smith H. O., Gibbs R. A., Myers E. W., Rubin G. M., Venter J. C. RT The genome sequence of Drosophila melanogaster RL Science 287:2185-2195 (2000). RN [2]; RE0015616. RX PUBMED: 9887103. RA Brummel T., Abdollah S., Haerry T. E., Shimell M. J., Merriam J., Raftery L., Wrana J. L., O'Connor M. B. RT The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development RL Genes Dev. 13:98-111 (1999). RN [3]; RE0015898. RX PUBMED: 7768443. RA Sekelsky J. J., Newfeld S. J., Raftery L. A., Chartoff E. H., Gelbart W. M. RT Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. RL Genetics 139:1347-1358 (1995). RN [4]; RE0015907. RX PUBMED: 9502724. RA Wisotzkey R. G., Mehra A., Sutherland D. J., Dobens L. L., Liu X., Dohrmann C., Attisano L., Raftery L. A. RT Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses. RL Development 125:1433-1445 (1998). RN [5]; RE0015915. RX PUBMED: 9693372. RA Inoue H., Imamura T., Ishidou Y., Takase M., Udagawa Y., Oka Y., Tsuneizumi K., Tabata T., Miyazono K., Kawabata M. RT Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp. RL Mol. Biol. Cell 9:2145-2156 (1998). RN [6]; RE0016203. RX PUBMED: 10884415. RA Xiao Z., Liu X., Henis Y. I., Lodish H. F. RT A distinct nuclear localization signal in the N terminus of Smad 3 determines its ligand-induced nuclear translocation. RL Proc. Natl. Acad. Sci. USA 97:7853-7858 (2000). RN [7]; RE0016241. RX PUBMED: 11071761. RA Dai H., Hogan C., Gopalakrishnan B., Torres-Vazquez J., Nguyen M., Park S., Raftery L. A., Warrior R., Arora K. RT The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic. RL Dev. Biol. 227:373-387 (2000). RN [8]; RE0016258. RX PUBMED: 10075933. RA Waltzer L., Bienz M. RT A function of CBP as a transcriptional co-activator during Dpp signalling. RL EMBO J. 18:1630-1641 (1999). RN [9]; RE0016261. RX PUBMED: 10320478. RA Das P., Inoue H., Baker J. C., Beppu H., Kawabata M., Harland R. M., Miyazono K., Padgett R. W. RT Drosophila dSmad2 and Atr-I transmit activin/TGFbeta signals. RL Genes Cells 4:123-134 (1999). RN [10]; RE0022036. RX PUBMED: 10370243. RA Zhang Y., Derynck R. RT Regulation of Smad signalling by protein associations and signalling crosstalk RL Trends Cell Biol. 9:274-279 (1999). RN [11]; RE0022065. RX PUBMED: 10712925. RA Attisano L., Wrana J. L. RT Smads as transcriptional co-modulators RL Curr. Opin. Cell Biol. 12:235-243 (2000). RN [12]; RE0022070. RX PUBMED: 9529613. RA Kretzschmar M., Massague J. RT SMADs: mediators and regulators of TGF-beta signaling RL Curr. Opin. Gen. Dev. 8:103-111 (1998). RN [13]; RE0022072. RX PUBMED: 10831835. RA Zimmerman C. M., Padgett R. W. RT Transforming growth factor beta signaling mediators and modulators RL Gene 249:17-30 (2000). XX //