
AC T01346
XX
ID T01346
XX
DT 25.10.1994 (created); ewi.
DT 29.10.2013 (updated); yre.
CO Copyright (C), QIAGEN.
XX
FA arnt-isoform1
XX
SY Ah receptor nuclear translocator; Arnt; aryl hydrocarbon receptor nuclear translocator; HIF-1beta; hypoxia-inducible factor 1beta.
XX
OS human, Homo sapiens
OC eukaryota; animalia; metazoa; chordata; vertebrata; tetrapoda; mammalia; eutheria; primates
XX
GE G004677 ARNT; HGNC: Arnt.
XX
CL C0010; bHLH; 1.2.5.2.1.1.
XX
SZ 789 AA; 86.6 kDa (cDNA) (calc.), 87 kDa (SDS) [4], 94 kDa (SDS) [2] [2] [4]
XX
SQ MAATTANPEMTSDVPSLGPAIASGNSGPGIQGGGAIVQRAIKRRPGLDFDDDGEGNSKFL
SQ RCDDDQMSNDKERFARSDDEQSSADKERLARENHSEIERRRRNKMTAYITELSDMVPTCS
SQ ALARKPDKLTILRMAVSHMKSLRGTGNTSTDGSYKPSFLTDQELKHLILEAADGFLFIVS
SQ CETGRVVYVSDSVTPVLNQPQSEWFGSTLYDQVHPDDVDKLREQLSTSENALTGRILDLK
SQ TGTVKKEGQQSSMRMCMGSRRSFICRMRCGSSSVDPVSVNRLSFVRNRCRNGLGSVKDGE
SQ PHFVVVHCTGYIKAWPPAGVSLPDDDPEAGQGSKFCLVAIGRLQVTSSPNCTDMSNVCQP
SQ TEFISRHNIEGIFTFVDHRCVATVGYQPQELLGKNIVEFCHPEDQQLLRDSFQQVVKLKG
SQ QVLSVMFRFRSKNQEWLWMRTSSFTFQNPYSDEIEYIICTNTNVKNSSQEPRPTLSNTIQ
SQ RPQLGPTANLPLEMGSGQLAPRQQQQQTELDMVPGRDGLASYNHSQVVQPVTTTGPEHSK
SQ PLEKSDGLFAQDRDPRFSEIYHNINADQSKGISSSTVPATQQLFSQGNTFPPTPRPAENF
SQ RNSGLAPPVTIVQPSASAGQMLAQISRHSNPTQGATPTWTPTTRSGFSAQQVATQATAKT
SQ RTSQFGVGSFQTPSSFSSMSLPGAPTASPGAAAYPSLTNRGSNFAPETGQTAGQFQTRTA
SQ EGVGVWPQWQGQQPHHRSSSSEQHVQQPPAQQPGQPEVFQEMLSMLGDQSNSYNNEEFPD
SQ LTMFPPFSE
XX
SC Swiss-Prot#P27540-1
XX
FT 85 143  PS50888; HLH.
FT 90 143
PS50888; HLH.
FT 90 143  PF00010; Helix-loop-helix DNA-binding domain.
FT 95 148
PF00010; Helix-loop-helix DNA-binding domain.
FT 95 148  SM00353; finulus.
FT 161 235
SM00353; finulus.
FT 161 235  PS50112; PAS.
FT 163 230
PS50112; PAS.
FT 163 230  SM00091; pas_2.
FT 163 270
SM00091; pas_2.
FT 163 270  PF00989; PAS fold.
FT 349 417
PF00989; PAS fold.
FT 349 417  SM00091; pas_2.
FT 352 464
SM00091; pas_2.
FT 352 464  PF00989; PAS fold.
FT 368 419
PF00989; PAS fold.
FT 368 419  PS50112; PAS.
FT 424 467
PS50112; PAS.
FT 424 467  SM00086; pac_2.
SM00086; pac_2.
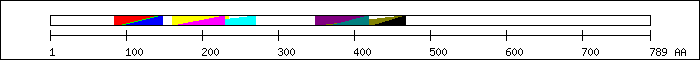 XX
SF there is a splice variant of 774 AA T01796 [1];
SF the PAS domain reveals some homology to Drosophila melanogaster Per and Sim [3];
SF in heterodimeric complexes with either AhR or Sim, arnt-isoform1 recognizes the 3'-half-site sequence GTG [3];
SF both the HLH and the PAS domain are required for homo- and heterodimerization (with HIF-1alpha) [8] [6];
SF in contrast to arnt-isoform1-AhR heterodimers, which also form in absence of DNA, arnt-isoform1 homodimers could only be observed in presence of binding site [3];
XX
EX blood,basophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,eosinophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,lymphocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,monocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,neutrophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX brain,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX brain,,,adult; low; Northern blot; total RNA; [8].
EX colon,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX colon,,,adult; high; Northern blot; total RNA; [8].
EX heart,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX heart,,,adult; high; Northern blot; total RNA; [8].
EX kidney (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX kidney (right and left),,,adult; low; Northern blot; total RNA; [8].
EX liver,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX liver,,,adult; low; Northern blot; total RNA; [8].
EX lung (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX lung (right and left),,,adult; low; Northern blot; total RNA; [8].
EX muscles,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX muscles,,,adult; very high; Northern blot; total RNA; [8].
EX ovary (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX ovary (right and left),,,adult; very high; Northern blot; total RNA; [8].
EX pancreas,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX pancreas,,,adult; high; Northern blot; total RNA; [8].
EX placenta,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX placenta,,,adult; very high; Northern blot; total RNA; [8].
EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX prostate gland,,,adult; low; Northern blot; total RNA; [8].
EX small intestine,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX small intestine,,,adult; low; Northern blot; total RNA; [8].
EX spleen,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX spleen,,,adult; high; Northern blot; total RNA; [8].
EX testis (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX testis (right and left),,,adult; very high; Northern blot; total RNA; [8].
EX thymus,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX thymus,,,adult; low; Northern blot; total RNA; [8].
XX
FF involved in and required for Ah receptor binding to XREs [4] [1];
FF no ligand (dioxin) binding;
FF strong constitutive activator [5];
FF relative contribution of arnt-isoform1 and AhR to overall activation by the AhR/arnt-isoform1 complex depend on cell type and promoter context [5];
FF HIF-1 (HIF-1alpha/arnt-isoform1) mediates gene responses to lowered oxygen levels (hypoxia, O2 demand exceeds supply) under which state the concentration of HIF-1beta (arnt-isoform1) mRNA and protein are increased [2];
FF HIF-1alpha competes with the ligand-activated AhR for binding to arnt-isoform1 (HIF-1beta) required for gene induction [8] [12];
XX
IN T00018 AhR; mouse, Mus musculus.
IN T01795 AhR; human, Homo sapiens.
IN T10385 Hey1-isoform1; human, Homo sapiens.
IN T09934 Hey2; human, Homo sapiens.
IN T01610 HIF-1alpha; human, Homo sapiens.
IN T02718 HIF2A; human, Homo sapiens.
IN T00373 HNF-4alpha1; human, Homo sapiens.
IN T00750 Sim; fruit fly, Drosophila melanogaster.
IN T04689 SMRT; human, Homo sapiens.
IN T00754 Sp1; rat, Rattus norvegicus.
IN T22417 SRC-1E; human, Homo sapiens.
IN T00818 TFIIB; human, Homo sapiens.
XX
MX M00235 V$AHRARNT_01.
MX M00237 V$AHRARNT_02.
MX M00976 V$AHRHIF_Q6.
MX M08868 V$ARNTLIKE_Q6.
MX M00236 V$ARNT_01.
MX M00539 V$ARNT_02.
MX M03812 V$ARNT_Q6.
MX M08800 V$ARNT_Q6_01.
MX M02378 V$HIF1AARNT_01.
XX
BS R00992.
BS R04653.
BS R04655.
BS R04654.
BS R12137.
BS R12138.
BS R12139.
BS R12140.
BS R12141.
BS R12142.
BS R12143.
BS R12144.
BS R12145.
BS R12146.
BS R12147.
BS R12148.
BS R12149.
BS R12150.
BS R12151.
BS R12152.
BS R12153.
BS R12154.
BS R12155.
BS R12156.
BS R12157.
BS R12158.
BS R12159.
BS R12160.
BS R12170.
BS R12171.
BS R12172.
BS R12173.
BS R12174.
BS R12175.
BS R12176.
BS R12177.
BS R12178.
BS R12179.
BS R12180.
BS R12181.
BS R12182.
BS R12183.
BS R12184.
BS R12185.
BS R12186.
BS R12187.
BS R12188.
BS R12189.
BS R12190.
BS R12191.
BS R12192.
BS R12193.
BS R12194.
BS R12195.
BS R13350.
BS R12196.
BS R12197.
BS R12198.
BS R12199.
BS R12200.
BS R12201.
BS R12202.
BS R12203.
BS R12204.
BS R12205.
BS R12206.
BS R12207.
BS R12208.
BS R12209.
BS R12210.
BS R12211.
BS R12212.
BS R12213.
BS R12214.
BS R12215.
BS R12216.
BS R12253.
BS R12254.
BS R12255.
BS R12256.
BS R12257.
BS R12258.
BS R12259.
BS R12260.
BS R12261.
BS R12262.
BS R12263.
BS R12264.
BS R12265.
BS R12266.
BS R12267.
BS R12268.
BS R12269.
BS R12270.
BS R12271.
BS R12272.
BS R12273.
BS R00272.
XX
DR TRANSPATH: MO000025604.
DR EMBL: M69238;
DR UniProtKB: P27540-1;
XX
RN [1]; RE0002644.
RX PUBMED: 1852076.
RA Hoffman E. C., Reyes H., Chu F.-F., Sander F., Conley H., Brooks B. A., Hankinson O.
RT Cloning of a factor required for activity of the Ah (dioxin) receptor
RL Science 252:954-958 (1991).
RN [2]; RE0003597.
RX PUBMED: 7539918.
RA Wang G. L., Jiang B.-H., Rue E. A., Semenza G. L.
RT Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension
RL Proc. Natl. Acad. Sci. USA 92:5510-5514 (1995).
RN [3]; RE0003789.
RX PUBMED: 7592839.
RA Swanson H. I., Chan W. K., Bradfield C. A.
RT DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins
RL J. Biol. Chem. 270:26292-26302 (1995).
RN [4]; RE0004045.
RX PUBMED: 1317062.
RA Reyes H., Reisz-Porszasz S., Hankinson O.
RT Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor
RL Science 256:1193-1195 (1992).
RN [5]; RE0004048.
RX PUBMED: 7969169.
RA Whitelaw M. L., Gustafsson J. A., Poellinger L.
RT Identification of transactivation and repression functions of the dioxin receptor and its basic helix-lop-helix/PAS partner Arnt: inductive versus constitutive modes of regulation
RL Mol. Cell. Biol. 14:8343-8355 (1994).
RN [6]; RE0005810.
RX PUBMED: 7892203.
RA Sogawa K., Nakano R., Kobayashi A., Kikuchi Y., Ohe N., Matsushita N., Kuriyama Y. F.
RT Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation
RL Proc. Natl. Acad. Sci. USA 92:1936-1940 (1995).
RN [7]; RE0008590.
RX PUBMED: 8407937.
RA Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y.
RT A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt
RL J. Biol. Chem. 268:21002- 21006 (1993).
RN [8]; RE0013068.
RX PUBMED: 8816435.
RA Gradin K., Guire J., Wenger R. H., Kvietikova I., Whitelaw M. L., Toftgard R., Tora L., Gassmann M., Poellinger L.
RT Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor
RL Mol. Cell. Biol. 16:5221-5231 (1996).
RN [9]; RE0017564.
RX PUBMED: 10454619.
RA Swanson H. I., Yang J. H.
RT Specificity of DNA binding of the c-Myc/Max and ARNT/ARNT dimers at the CACGTG recognition site.
RL Nucleic Acids Res. 27:3205-3212 (1999).
RN [10]; RE0021482.
RX PUBMED: 12097158.
RA Tsuchiya T., Kominato Y., Ueda M.
RT Human Hypoxic Signal Transduction through a Signature Motif in Hepatocyte Nuclear Factor 4
RL J. Biochem. 132:37-44 (2002).
RN [11]; RE0022293.
RX PUBMED: 11593383.
RA Suzuki H., Tomida A., Tsuruo T.
RT Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia.
RL Oncogene 20:5779-5788 (2001).
RN [12]; RE0022396.
RX PUBMED: 10207038.
RA Chan W. K., Yao G., Gu Y. Z., Bradfield C. A.
RT Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation.
RL J. Biol. Chem. 274:12115-12123 (1999).
XX
//
XX
SF there is a splice variant of 774 AA T01796 [1];
SF the PAS domain reveals some homology to Drosophila melanogaster Per and Sim [3];
SF in heterodimeric complexes with either AhR or Sim, arnt-isoform1 recognizes the 3'-half-site sequence GTG [3];
SF both the HLH and the PAS domain are required for homo- and heterodimerization (with HIF-1alpha) [8] [6];
SF in contrast to arnt-isoform1-AhR heterodimers, which also form in absence of DNA, arnt-isoform1 homodimers could only be observed in presence of binding site [3];
XX
EX blood,basophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,eosinophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,lymphocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,monocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX blood,neutrophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8].
EX brain,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX brain,,,adult; low; Northern blot; total RNA; [8].
EX colon,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX colon,,,adult; high; Northern blot; total RNA; [8].
EX heart,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX heart,,,adult; high; Northern blot; total RNA; [8].
EX kidney (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX kidney (right and left),,,adult; low; Northern blot; total RNA; [8].
EX liver,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX liver,,,adult; low; Northern blot; total RNA; [8].
EX lung (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX lung (right and left),,,adult; low; Northern blot; total RNA; [8].
EX muscles,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX muscles,,,adult; very high; Northern blot; total RNA; [8].
EX ovary (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX ovary (right and left),,,adult; very high; Northern blot; total RNA; [8].
EX pancreas,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX pancreas,,,adult; high; Northern blot; total RNA; [8].
EX placenta,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX placenta,,,adult; very high; Northern blot; total RNA; [8].
EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX prostate gland,,,adult; low; Northern blot; total RNA; [8].
EX small intestine,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX small intestine,,,adult; low; Northern blot; total RNA; [8].
EX spleen,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX spleen,,,adult; high; Northern blot; total RNA; [8].
EX testis (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8].
EX testis (right and left),,,adult; very high; Northern blot; total RNA; [8].
EX thymus,,,adult; detectable; Northern blot; RNA (undefined); [8].
EX thymus,,,adult; low; Northern blot; total RNA; [8].
XX
FF involved in and required for Ah receptor binding to XREs [4] [1];
FF no ligand (dioxin) binding;
FF strong constitutive activator [5];
FF relative contribution of arnt-isoform1 and AhR to overall activation by the AhR/arnt-isoform1 complex depend on cell type and promoter context [5];
FF HIF-1 (HIF-1alpha/arnt-isoform1) mediates gene responses to lowered oxygen levels (hypoxia, O2 demand exceeds supply) under which state the concentration of HIF-1beta (arnt-isoform1) mRNA and protein are increased [2];
FF HIF-1alpha competes with the ligand-activated AhR for binding to arnt-isoform1 (HIF-1beta) required for gene induction [8] [12];
XX
IN T00018 AhR; mouse, Mus musculus.
IN T01795 AhR; human, Homo sapiens.
IN T10385 Hey1-isoform1; human, Homo sapiens.
IN T09934 Hey2; human, Homo sapiens.
IN T01610 HIF-1alpha; human, Homo sapiens.
IN T02718 HIF2A; human, Homo sapiens.
IN T00373 HNF-4alpha1; human, Homo sapiens.
IN T00750 Sim; fruit fly, Drosophila melanogaster.
IN T04689 SMRT; human, Homo sapiens.
IN T00754 Sp1; rat, Rattus norvegicus.
IN T22417 SRC-1E; human, Homo sapiens.
IN T00818 TFIIB; human, Homo sapiens.
XX
MX M00235 V$AHRARNT_01.
MX M00237 V$AHRARNT_02.
MX M00976 V$AHRHIF_Q6.
MX M08868 V$ARNTLIKE_Q6.
MX M00236 V$ARNT_01.
MX M00539 V$ARNT_02.
MX M03812 V$ARNT_Q6.
MX M08800 V$ARNT_Q6_01.
MX M02378 V$HIF1AARNT_01.
XX
BS R00992.
BS R04653.
BS R04655.
BS R04654.
BS R12137.
BS R12138.
BS R12139.
BS R12140.
BS R12141.
BS R12142.
BS R12143.
BS R12144.
BS R12145.
BS R12146.
BS R12147.
BS R12148.
BS R12149.
BS R12150.
BS R12151.
BS R12152.
BS R12153.
BS R12154.
BS R12155.
BS R12156.
BS R12157.
BS R12158.
BS R12159.
BS R12160.
BS R12170.
BS R12171.
BS R12172.
BS R12173.
BS R12174.
BS R12175.
BS R12176.
BS R12177.
BS R12178.
BS R12179.
BS R12180.
BS R12181.
BS R12182.
BS R12183.
BS R12184.
BS R12185.
BS R12186.
BS R12187.
BS R12188.
BS R12189.
BS R12190.
BS R12191.
BS R12192.
BS R12193.
BS R12194.
BS R12195.
BS R13350.
BS R12196.
BS R12197.
BS R12198.
BS R12199.
BS R12200.
BS R12201.
BS R12202.
BS R12203.
BS R12204.
BS R12205.
BS R12206.
BS R12207.
BS R12208.
BS R12209.
BS R12210.
BS R12211.
BS R12212.
BS R12213.
BS R12214.
BS R12215.
BS R12216.
BS R12253.
BS R12254.
BS R12255.
BS R12256.
BS R12257.
BS R12258.
BS R12259.
BS R12260.
BS R12261.
BS R12262.
BS R12263.
BS R12264.
BS R12265.
BS R12266.
BS R12267.
BS R12268.
BS R12269.
BS R12270.
BS R12271.
BS R12272.
BS R12273.
BS R00272.
XX
DR TRANSPATH: MO000025604.
DR EMBL: M69238;
DR UniProtKB: P27540-1;
XX
RN [1]; RE0002644.
RX PUBMED: 1852076.
RA Hoffman E. C., Reyes H., Chu F.-F., Sander F., Conley H., Brooks B. A., Hankinson O.
RT Cloning of a factor required for activity of the Ah (dioxin) receptor
RL Science 252:954-958 (1991).
RN [2]; RE0003597.
RX PUBMED: 7539918.
RA Wang G. L., Jiang B.-H., Rue E. A., Semenza G. L.
RT Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension
RL Proc. Natl. Acad. Sci. USA 92:5510-5514 (1995).
RN [3]; RE0003789.
RX PUBMED: 7592839.
RA Swanson H. I., Chan W. K., Bradfield C. A.
RT DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins
RL J. Biol. Chem. 270:26292-26302 (1995).
RN [4]; RE0004045.
RX PUBMED: 1317062.
RA Reyes H., Reisz-Porszasz S., Hankinson O.
RT Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor
RL Science 256:1193-1195 (1992).
RN [5]; RE0004048.
RX PUBMED: 7969169.
RA Whitelaw M. L., Gustafsson J. A., Poellinger L.
RT Identification of transactivation and repression functions of the dioxin receptor and its basic helix-lop-helix/PAS partner Arnt: inductive versus constitutive modes of regulation
RL Mol. Cell. Biol. 14:8343-8355 (1994).
RN [6]; RE0005810.
RX PUBMED: 7892203.
RA Sogawa K., Nakano R., Kobayashi A., Kikuchi Y., Ohe N., Matsushita N., Kuriyama Y. F.
RT Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation
RL Proc. Natl. Acad. Sci. USA 92:1936-1940 (1995).
RN [7]; RE0008590.
RX PUBMED: 8407937.
RA Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y.
RT A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt
RL J. Biol. Chem. 268:21002- 21006 (1993).
RN [8]; RE0013068.
RX PUBMED: 8816435.
RA Gradin K., Guire J., Wenger R. H., Kvietikova I., Whitelaw M. L., Toftgard R., Tora L., Gassmann M., Poellinger L.
RT Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor
RL Mol. Cell. Biol. 16:5221-5231 (1996).
RN [9]; RE0017564.
RX PUBMED: 10454619.
RA Swanson H. I., Yang J. H.
RT Specificity of DNA binding of the c-Myc/Max and ARNT/ARNT dimers at the CACGTG recognition site.
RL Nucleic Acids Res. 27:3205-3212 (1999).
RN [10]; RE0021482.
RX PUBMED: 12097158.
RA Tsuchiya T., Kominato Y., Ueda M.
RT Human Hypoxic Signal Transduction through a Signature Motif in Hepatocyte Nuclear Factor 4
RL J. Biochem. 132:37-44 (2002).
RN [11]; RE0022293.
RX PUBMED: 11593383.
RA Suzuki H., Tomida A., Tsuruo T.
RT Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia.
RL Oncogene 20:5779-5788 (2001).
RN [12]; RE0022396.
RX PUBMED: 10207038.
RA Chan W. K., Yao G., Gu Y. Z., Bradfield C. A.
RT Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation.
RL J. Biol. Chem. 274:12115-12123 (1999).
XX
//


PS50888; HLH. FT 90 143
PF00010; Helix-loop-helix DNA-binding domain. FT 95 148
SM00353; finulus. FT 161 235
PS50112; PAS. FT 163 230
SM00091; pas_2. FT 163 270
PF00989; PAS fold. FT 349 417
SM00091; pas_2. FT 352 464
PF00989; PAS fold. FT 368 419
PS50112; PAS. FT 424 467
SM00086; pac_2.
XX SF there is a splice variant of 774 AA T01796 [1]; SF the PAS domain reveals some homology to Drosophila melanogaster Per and Sim [3]; SF in heterodimeric complexes with either AhR or Sim, arnt-isoform1 recognizes the 3'-half-site sequence GTG [3]; SF both the HLH and the PAS domain are required for homo- and heterodimerization (with HIF-1alpha) [8] [6]; SF in contrast to arnt-isoform1-AhR heterodimers, which also form in absence of DNA, arnt-isoform1 homodimers could only be observed in presence of binding site [3]; XX EX blood,basophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8]. EX blood,eosinophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8]. EX blood,lymphocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8]. EX blood,monocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8]. EX blood,neutrophil granulocyte,Circulatory System & Hematopoietic System,adult; very low; Northern blot; total RNA; [8]. EX brain,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX brain,,,adult; low; Northern blot; total RNA; [8]. EX colon,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX colon,,,adult; high; Northern blot; total RNA; [8]. EX heart,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX heart,,,adult; high; Northern blot; total RNA; [8]. EX kidney (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8]. EX kidney (right and left),,,adult; low; Northern blot; total RNA; [8]. EX liver,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX liver,,,adult; low; Northern blot; total RNA; [8]. EX lung (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8]. EX lung (right and left),,,adult; low; Northern blot; total RNA; [8]. EX muscles,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX muscles,,,adult; very high; Northern blot; total RNA; [8]. EX ovary (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8]. EX ovary (right and left),,,adult; very high; Northern blot; total RNA; [8]. EX pancreas,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX pancreas,,,adult; high; Northern blot; total RNA; [8]. EX placenta,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX placenta,,,adult; very high; Northern blot; total RNA; [8]. EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX prostate gland,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX prostate gland,,,adult; low; Northern blot; total RNA; [8]. EX small intestine,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX small intestine,,,adult; low; Northern blot; total RNA; [8]. EX spleen,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX spleen,,,adult; high; Northern blot; total RNA; [8]. EX testis (right and left),,,adult; detectable; Northern blot; RNA (undefined); [8]. EX testis (right and left),,,adult; very high; Northern blot; total RNA; [8]. EX thymus,,,adult; detectable; Northern blot; RNA (undefined); [8]. EX thymus,,,adult; low; Northern blot; total RNA; [8]. XX FF involved in and required for Ah receptor binding to XREs [4] [1]; FF no ligand (dioxin) binding; FF strong constitutive activator [5]; FF relative contribution of arnt-isoform1 and AhR to overall activation by the AhR/arnt-isoform1 complex depend on cell type and promoter context [5]; FF HIF-1 (HIF-1alpha/arnt-isoform1) mediates gene responses to lowered oxygen levels (hypoxia, O2 demand exceeds supply) under which state the concentration of HIF-1beta (arnt-isoform1) mRNA and protein are increased [2]; FF HIF-1alpha competes with the ligand-activated AhR for binding to arnt-isoform1 (HIF-1beta) required for gene induction [8] [12]; XX IN T00018 AhR; mouse, Mus musculus. IN T01795 AhR; human, Homo sapiens. IN T10385 Hey1-isoform1; human, Homo sapiens. IN T09934 Hey2; human, Homo sapiens. IN T01610 HIF-1alpha; human, Homo sapiens. IN T02718 HIF2A; human, Homo sapiens. IN T00373 HNF-4alpha1; human, Homo sapiens. IN T00750 Sim; fruit fly, Drosophila melanogaster. IN T04689 SMRT; human, Homo sapiens. IN T00754 Sp1; rat, Rattus norvegicus. IN T22417 SRC-1E; human, Homo sapiens. IN T00818 TFIIB; human, Homo sapiens. XX MX M00235 V$AHRARNT_01. MX M00237 V$AHRARNT_02. MX M00976 V$AHRHIF_Q6. MX M08868 V$ARNTLIKE_Q6. MX M00236 V$ARNT_01. MX M00539 V$ARNT_02. MX M03812 V$ARNT_Q6. MX M08800 V$ARNT_Q6_01. MX M02378 V$HIF1AARNT_01. XX BS R00992. BS R04653. BS R04655. BS R04654. BS R12137. BS R12138. BS R12139. BS R12140. BS R12141. BS R12142. BS R12143. BS R12144. BS R12145. BS R12146. BS R12147. BS R12148. BS R12149. BS R12150. BS R12151. BS R12152. BS R12153. BS R12154. BS R12155. BS R12156. BS R12157. BS R12158. BS R12159. BS R12160. BS R12170. BS R12171. BS R12172. BS R12173. BS R12174. BS R12175. BS R12176. BS R12177. BS R12178. BS R12179. BS R12180. BS R12181. BS R12182. BS R12183. BS R12184. BS R12185. BS R12186. BS R12187. BS R12188. BS R12189. BS R12190. BS R12191. BS R12192. BS R12193. BS R12194. BS R12195. BS R13350. BS R12196. BS R12197. BS R12198. BS R12199. BS R12200. BS R12201. BS R12202. BS R12203. BS R12204. BS R12205. BS R12206. BS R12207. BS R12208. BS R12209. BS R12210. BS R12211. BS R12212. BS R12213. BS R12214. BS R12215. BS R12216. BS R12253. BS R12254. BS R12255. BS R12256. BS R12257. BS R12258. BS R12259. BS R12260. BS R12261. BS R12262. BS R12263. BS R12264. BS R12265. BS R12266. BS R12267. BS R12268. BS R12269. BS R12270. BS R12271. BS R12272. BS R12273. BS R00272. XX DR TRANSPATH: MO000025604. DR EMBL: M69238; DR UniProtKB: P27540-1; XX RN [1]; RE0002644. RX PUBMED: 1852076. RA Hoffman E. C., Reyes H., Chu F.-F., Sander F., Conley H., Brooks B. A., Hankinson O. RT Cloning of a factor required for activity of the Ah (dioxin) receptor RL Science 252:954-958 (1991). RN [2]; RE0003597. RX PUBMED: 7539918. RA Wang G. L., Jiang B.-H., Rue E. A., Semenza G. L. RT Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension RL Proc. Natl. Acad. Sci. USA 92:5510-5514 (1995). RN [3]; RE0003789. RX PUBMED: 7592839. RA Swanson H. I., Chan W. K., Bradfield C. A. RT DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins RL J. Biol. Chem. 270:26292-26302 (1995). RN [4]; RE0004045. RX PUBMED: 1317062. RA Reyes H., Reisz-Porszasz S., Hankinson O. RT Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor RL Science 256:1193-1195 (1992). RN [5]; RE0004048. RX PUBMED: 7969169. RA Whitelaw M. L., Gustafsson J. A., Poellinger L. RT Identification of transactivation and repression functions of the dioxin receptor and its basic helix-lop-helix/PAS partner Arnt: inductive versus constitutive modes of regulation RL Mol. Cell. Biol. 14:8343-8355 (1994). RN [6]; RE0005810. RX PUBMED: 7892203. RA Sogawa K., Nakano R., Kobayashi A., Kikuchi Y., Ohe N., Matsushita N., Kuriyama Y. F. RT Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation RL Proc. Natl. Acad. Sci. USA 92:1936-1940 (1995). RN [7]; RE0008590. RX PUBMED: 8407937. RA Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y. RT A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt RL J. Biol. Chem. 268:21002- 21006 (1993). RN [8]; RE0013068. RX PUBMED: 8816435. RA Gradin K., Guire J., Wenger R. H., Kvietikova I., Whitelaw M. L., Toftgard R., Tora L., Gassmann M., Poellinger L. RT Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor RL Mol. Cell. Biol. 16:5221-5231 (1996). RN [9]; RE0017564. RX PUBMED: 10454619. RA Swanson H. I., Yang J. H. RT Specificity of DNA binding of the c-Myc/Max and ARNT/ARNT dimers at the CACGTG recognition site. RL Nucleic Acids Res. 27:3205-3212 (1999). RN [10]; RE0021482. RX PUBMED: 12097158. RA Tsuchiya T., Kominato Y., Ueda M. RT Human Hypoxic Signal Transduction through a Signature Motif in Hepatocyte Nuclear Factor 4 RL J. Biochem. 132:37-44 (2002). RN [11]; RE0022293. RX PUBMED: 11593383. RA Suzuki H., Tomida A., Tsuruo T. RT Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. RL Oncogene 20:5779-5788 (2001). RN [12]; RE0022396. RX PUBMED: 10207038. RA Chan W. K., Yao G., Gu Y. Z., Bradfield C. A. RT Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. RL J. Biol. Chem. 274:12115-12123 (1999). XX //